MSDS Flexcera Base Ultra+
Material datasheet (MSDS) Flexcera Base Ultra+
Read More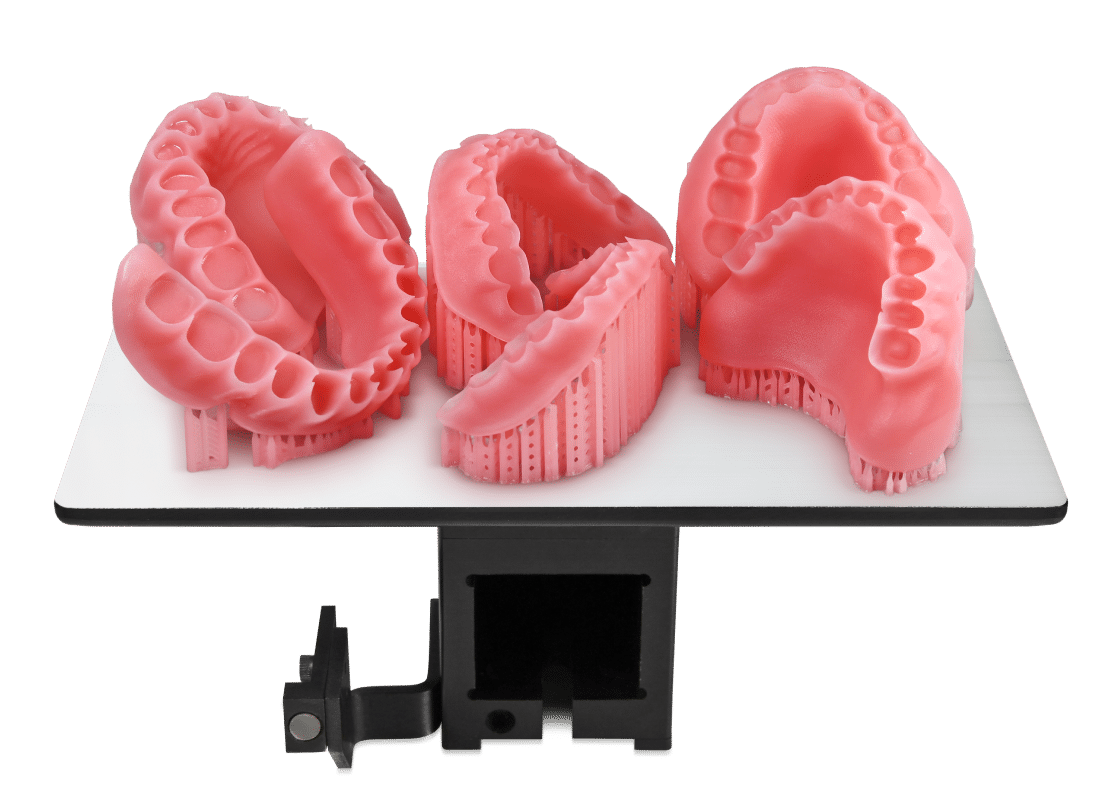
Desktop Health®
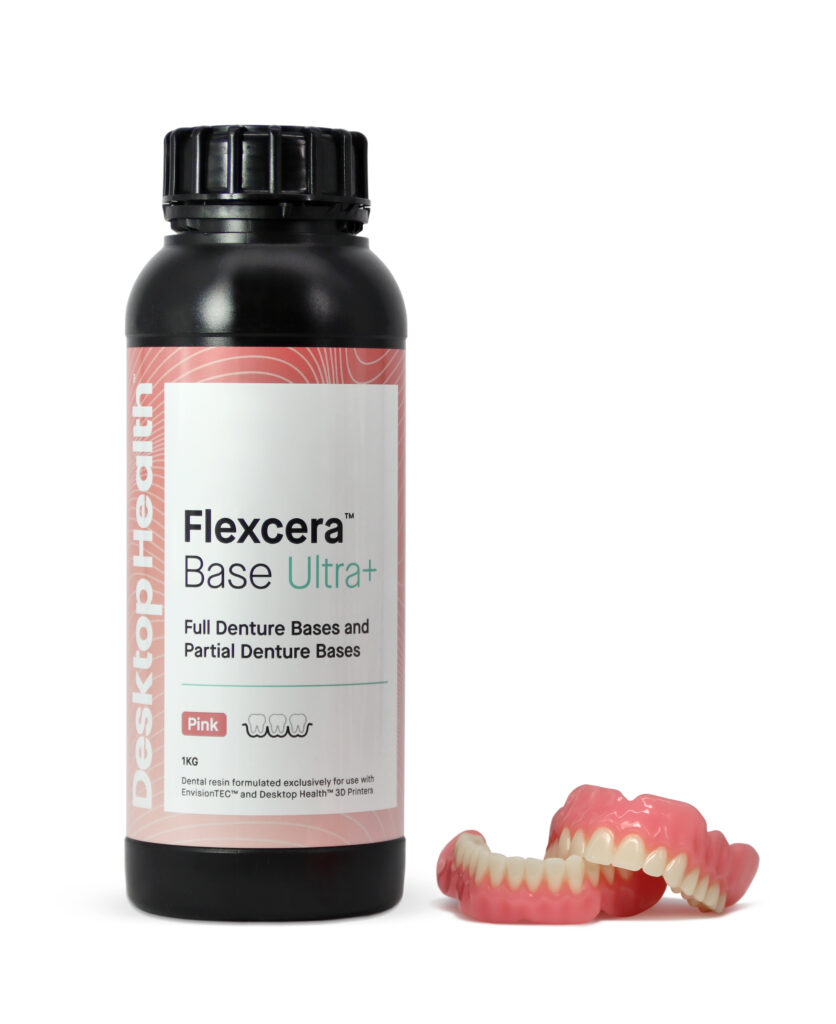
Flexcera Base™ is a light-curable resin for the fabrication of high-impact and removable denture bases. It's an FDA 510(k) Cleared Class 2 Medical Device indicated for the fabrication of denture bases in dental laboratories for full removable dentures and formulated exclusively for use with the Einstein or EnvisionTEC™ 3D printers.
Flexcera™ is a new FDA-approved resin used by professionals in the dental industry to create beautiful, functional dentures with ceramic-like strength produced at 3D printed speed.

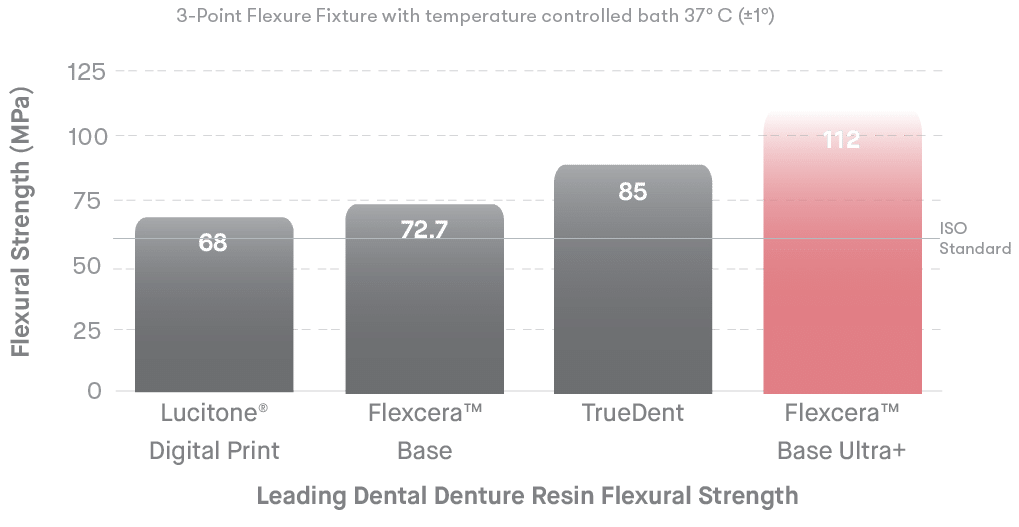
The Flexcera formulation provides ceramic-like strength and is designed for long-term use.
Flexcera™ Base Ultra+ is an FDA 510(k) Class II cleared, CE Marked, and MDR Class I certified medical device for the additive manufacturing of denture bases for full and partial removable dentures.
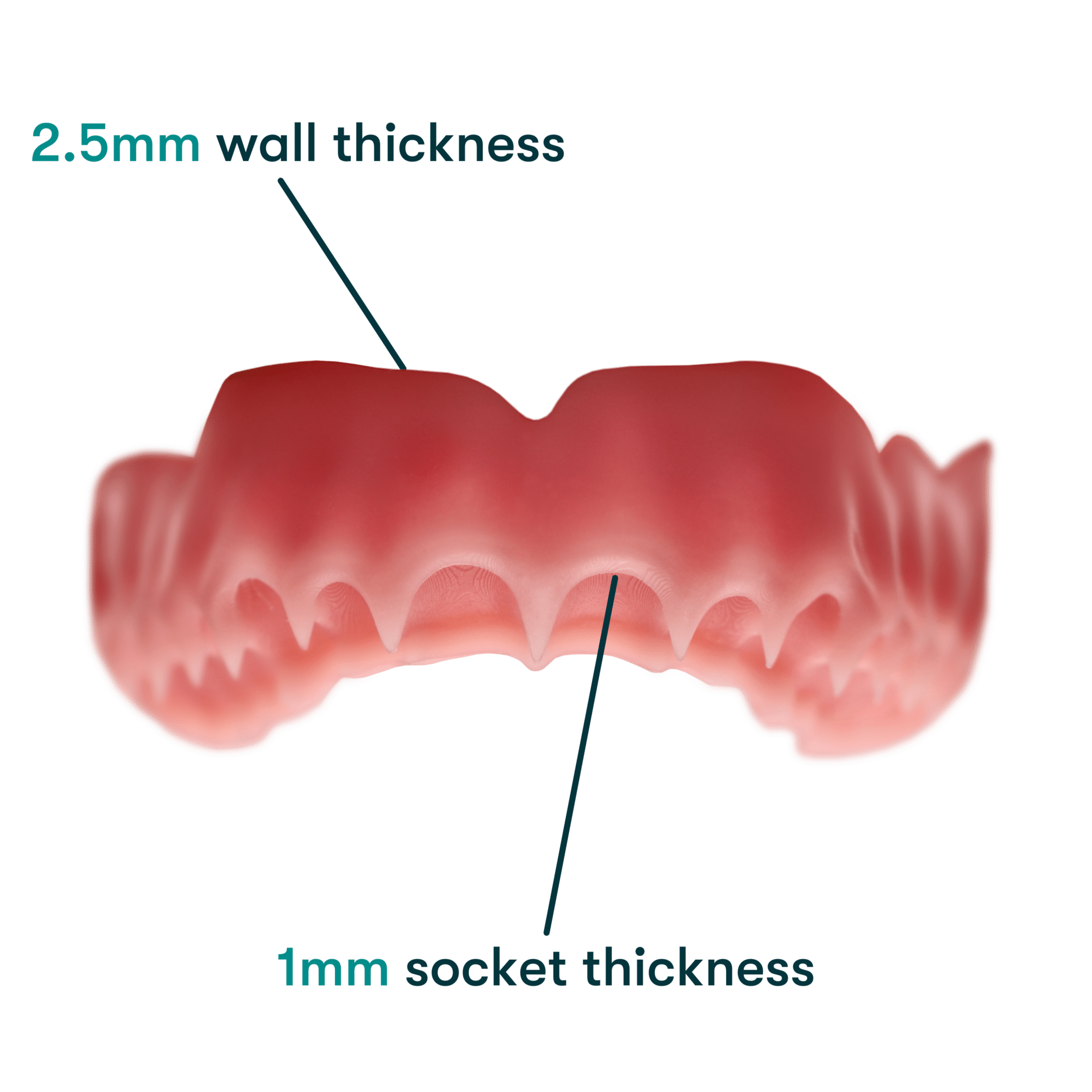
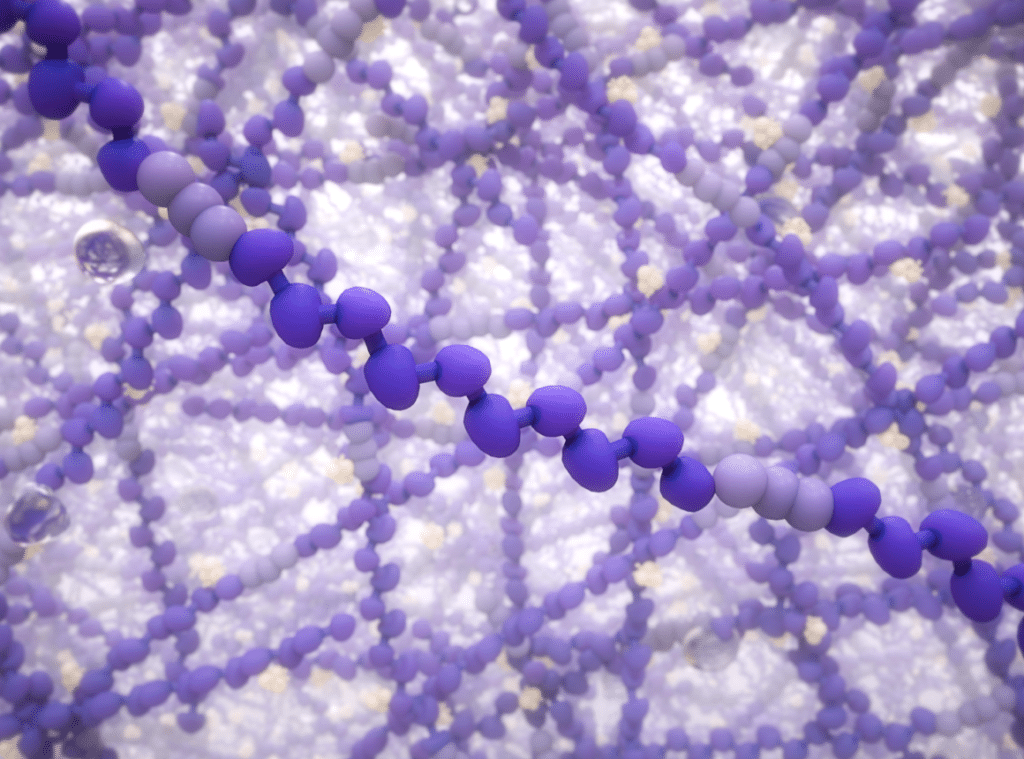
Thanks to Desktop Health’s long-chain chemistry, Flexcera Base Ultra+ delivers a stronger and stiffer denture base for thinner designs. It boasts a 70% greater resistance to deformation compared to the ISO standard.
Material datasheet (MSDS) Flexcera Base Ultra+
Read MoreMaterial Datasheet (MSDS) Flexcera Base™
Read MoreDesktop Health Patient Care Instructions
Read MoreFlexcera Base™ | Instructions for Use (IFU)
Read More