E-Guide | Instructions for Use
The E-Guide Instructions for Use (IFU)
Read MoreBiocompatible 3D printing material for surgical drill guides

Desktop Health™
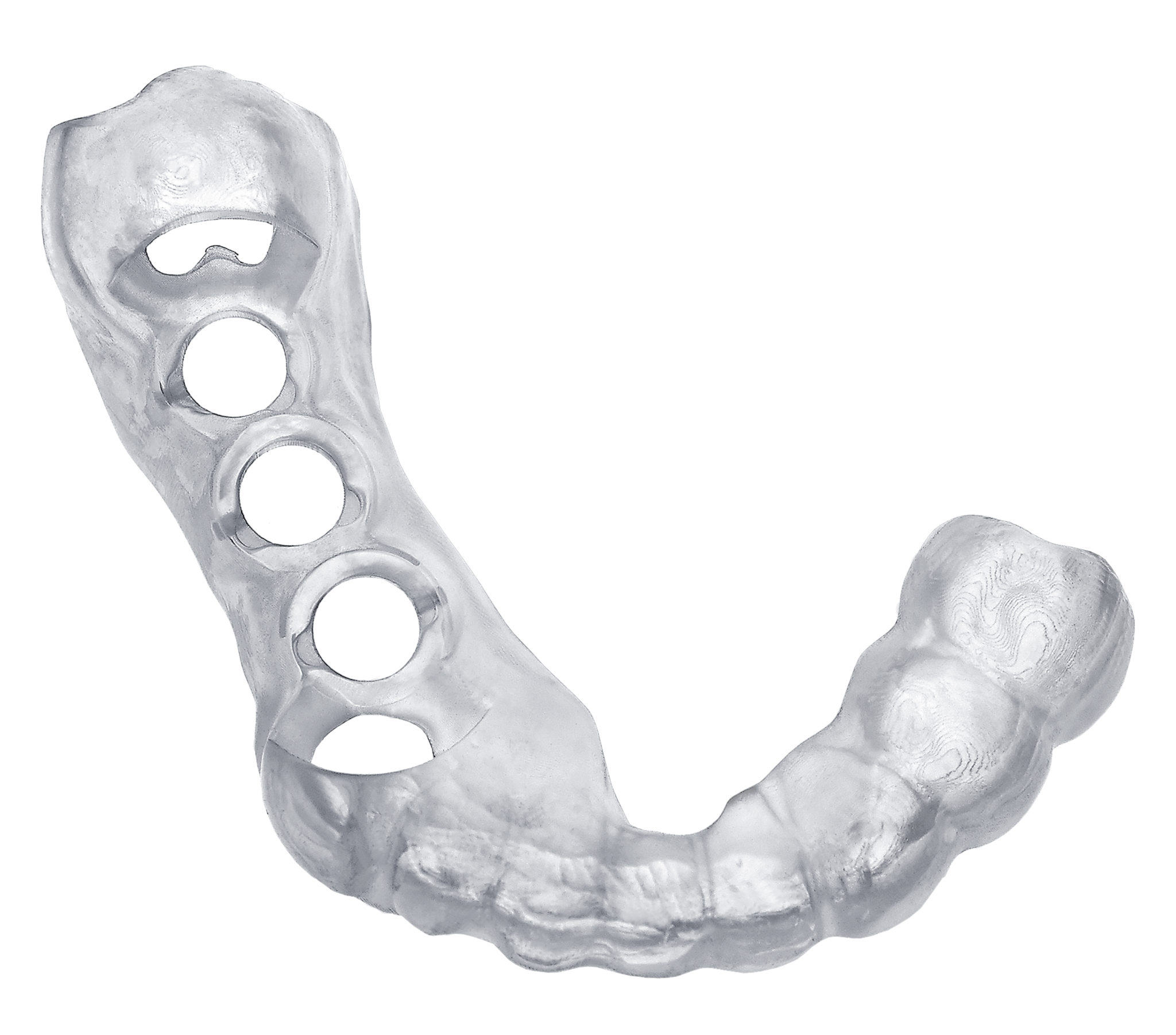
E-Guide is an FDA certified Class I material, developed for the production of high-precision surgical drill guides for use in implant surgery. The results produced by combining E-Guide with EnvisionTEC's cDLP technology are superior to traditional methods of manufacturing implant placement guides.
High-resolution 3D printing allows for precise targeted placement of implants during surgery. Drill sleeves may be inserted directly after printing, with excellent fit. E-Guide is registered with the FDA as a Class I medical device and is approved by Health Canada for its use as a surgical guide material.
The cost of high-quality drill guides can be a lot lower when using E-Guide on an EnvisionTEC 3D printer in comparison to previous machining methods.
Ultimate Flexural Strength |
Ultimate Flexural Strength79 - 85 MPaMethod: DIN EN ISO 20795-2:2013 |
Flexural Modulus |
Flexural Modulus2030 - 2059 MPaMethod: DIN EN ISO 20795-2:2013 |
Water Sorption |
Water Sorption30 - 32 µm/mm3Method: DIN EN ISO 20795-2:2013 |
Water Solubility |
Water Solubility0.5 µm/mm3Method: DIN EN ISO 20795-2:2013 |
Viscosity @ 30°C |
Viscosity @ 30°C230 - 330 cPBrookfield Method |
Cytotoxicity |
CytotoxicityPassedISO 10993-5 |
Irritation and skin sensitization |
Irritation and skin sensitizationPassedISO 10993-10 |
Speak to an expert to understand why DLP 3D printing processes and technologies
are a game-changer for the dental industry
The E-Guide Instructions for Use (IFU)
Read MoreSafety Data Sheet according to Regulation (EC) No. 1907/2006 (REACH) Photopolymer E-Guide - USA, EGuide, E-Guide 2.0, E-Guide Blue
Read MoreEnvisionTEC’s E-Guide is a biocompatible certified Class I material, developed for the production of high precision surgical drill guides for use in implant surgery
Read More